Answer:
Step-by-step explanation:
Potassium carbonate is a used in the production of cocoa powder. It is used to enhance the aroma and to produce Dutch processed chocolate. It is used to reduce acidity of the natural cocoa beans. The process of the addition of potassium carbonate to cocoa powder is known as Dutching.
The balanced reaction of potassium carbonate with any acid, say HA is shown below:
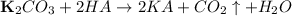
Potassium carbonate on reaction with an acid give the corresponding salt of potassium and water and carbon dioxide gas is evolved out.