Answer: The equations are given below.
Step-by-step explanation:
For the given options:
- Option 1:
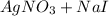
The molecular equation for this follows:
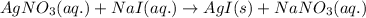
Ionic form of the above equation follows:
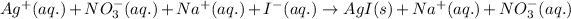
As, sodium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation.
The net ionic equation for the above reaction follows:
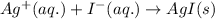
- Option 2:
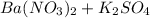
The molecular equation for this follows:
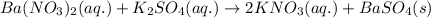
Ionic form of the above equation follows:
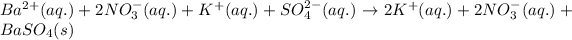
As, potassium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation.
The net ionic equation for the above reaction follows:
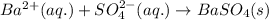
- Option 3:
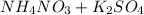
The molecular equation for this follows:
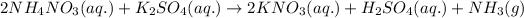
Ionic form of the above equation follows:

As, nitrate, potassium and sulfate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation.
The net ionic equation for the above reaction follows:
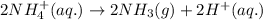
- Option 4:
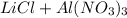
The molecular equation for this follows:
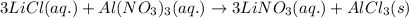
Ionic form of the above equation follows:
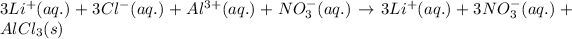
As, lithium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation.
The net ionic equation for the above reaction follows:
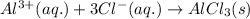
Hence, the molecular and ionic equations are given above.