Answer-
The molar concentration of
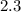 g NaCl glucose in
g NaCl glucose in
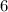 liters of solution is
liters of solution is
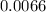 moles/litre
moles/litre
Step-by-step explanation:
Molarity is the number of moles of a substance in a unit volume of solution. Molarity is known as molar concentration and its unit is
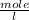
Number of moles in
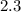 grams of NaCL
grams of NaCL
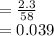
Molar concentration
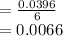 moles/litre
moles/litre
Hence, the molar concentration of
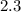 g NaCl glucose in
g NaCl glucose in
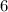 liters of solution is
liters of solution is
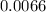 moles/litre
moles/litre