Answer: lambda
 , nu
, nu
 , and 100E
, and 100E
Step-by-step explanation:
The energy
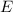 of a photon is given by:
of a photon is given by:
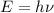 (1)
(1)
Where:
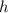 is the Planck constant
is the Planck constant
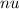 is the frequency
is the frequency
On the other hand, we have an expression that relates the frequency of the photn with its wavelength
 :
:
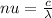 (2) where
(2) where
 is the speed of light
is the speed of light
Substituting (2) in (1):
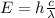 (3) This is the energy for a single photon
(3) This is the energy for a single photon
For 100 photons, the energy is:
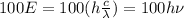 (3)
(3)
Where the wavelength and the frequency of the light remains constant.
Therefore, the answer is:
 ,
,
 , and 100E
, and 100E