Answer:
Approximately 0.074 grams.
Step-by-step explanation:
- Look up the relative atomic mass of copper on a modern periodic table:
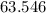 .
. - Look up the Faraday's Constant:
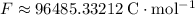 . This constant gives the amount of electrical charge on each mole of electrons.
. This constant gives the amount of electrical charge on each mole of electrons.
How does the electroplating works for copper? Copper exists as copper(II) ions
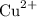 in the copper(II) nitrate
in the copper(II) nitrate
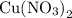 solution. It takes two moles of electrons to reduce one mole of copper(II) ions
solution. It takes two moles of electrons to reduce one mole of copper(II) ions
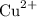 to metallic copper
to metallic copper
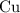 .
.
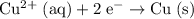 . (Reduction half of the ionic equation.)
. (Reduction half of the ionic equation.)
What are the steps for finding the mass of copper that has been deposited.
Start by finding the charge on the electrons that have been supplied to this electrochemical cell. After that,
- Find the number of moles of electrons that have been supplied based on the charge supplied;
- Find the number of moles of copper that have been reduced based on the number of moles of electrons supplied; and
- Find the mass of copper based on the number of moles of copper atoms reduced.
What's the charge
 on the electrons supplied to this electrochemical cell?
on the electrons supplied to this electrochemical cell?
For a constant direct current
 , the charge
, the charge
 that has been delivered in time
that has been delivered in time
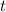 is equal to
is equal to
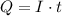 .
.
In case
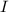 is in Amperes
is in Amperes
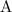 and
and
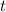 is in seconds
is in seconds
 ,
,
 will be in Coulombs
will be in Coulombs
 (which is the same as
(which is the same as
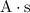 .)
.)
For this electrochemical cell,
 .
.
How many moles of electrons were supplied to this electrochemical cell?
The Faraday's Constant gives the size of charge (in Coulombs) on one mole of electrons.
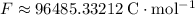 .
.
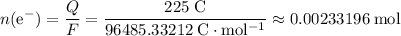 .
.
How many moles of copper atoms have been deposited?
Assume that copper(II) ions
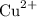 are in excess. Refer to the reduction half-equation, it takes two moles of electrons to deposit one mole of metallic copper.
are in excess. Refer to the reduction half-equation, it takes two moles of electrons to deposit one mole of metallic copper.
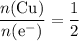 .
.
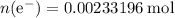 . As a result,
. As a result,
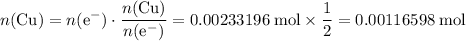 .
.
What's the mass of that many copper atoms?
The Relative atomic mass of copper is
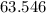 .
.
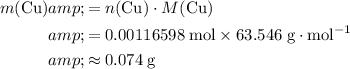 .
.