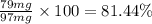Answer:
The percent recovery from re crystallization for both compounds A and B is 69.745 and 81.44 % respectively.
Step-by-step explanation:
Mass of compound A in a mixture = 119 mg
Mass of compound A after re-crystallization = 83 mg
Percent recovery from re-crystallization :
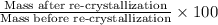
Percent recovery of compound A:
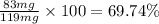
Mass of compound B in a mixture = 97 mg
Mass of compound B after re-crystallization = 79 mg
Percent recovery of compound B: