Answer: The correct answer is Aufbau principle.
Step-by-step explanation:
Pauli exclusion principle states that no two electrons with in an atom can have all four quantum numbers same.
Heisenberg principle states that it is impossible to measure with high precision the value of momentum and position of an electron.
Aufbau principle states that the electron will occupy the lowest energy level first before occupying the higher energy levels.
Schrodinger equation is used to find the allowed energy levels of quantum mechanical systems of an electron.
Chromium is the 24th element of the periodic table and its electronic configuration must be written as:
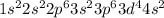
But the actual configuration for this is
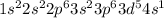
This configuration is an exception to Aufbau's principle because half filled sub-levels is more stable than other configurations.
The actual configuration has half filled 'd' and 's' sub-levels but the expected configuration had fully filled 's' orbital and partially filled 'd' orbital.
Thus, actual configuration is accepted for chromium atom.
Hence, the correct answer is Aufbau's principle.