Answer : The answer in standard form is, 0.00318 mg.
Explanation :
Significant figures : The figures in a number which express the value -the magnitude of a quantity to a specific degree of accuracy is known as significant digits.
The rule apply for the addition and subtraction is :
The least precise number present after the decimal point determines the number of significant figures in the answer.
As we are given :

First we have to convert scientific notation into standard form.
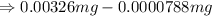
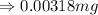
As per rule, the least precise number present after the decimal point is 5. So, the answer will be, 0.00318 mg.