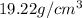Answer: The density of gold in
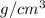 is
is
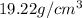
Step-by-step explanation:
Density is defined as the ratio of mass of the object and volume of the object. Mathematically,
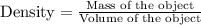
We are given:
Density of gold =
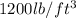
Using conversion factors:
1 lb = 453.6 g
1 feet = 12 inches
1 inch = 2.54 cm
Converting given quantity into
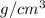 , we get:
, we get:
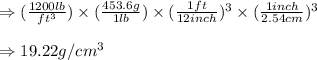
Hence, the density of gold in
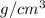 is
is