Answer : The pH of a saturated solution is, 12.33
Explanation : Given,
 =
=
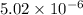
First we have to calculate the solubility of
 ion.
ion.
The balanced equilibrium reaction will be:
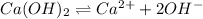
Let the solubility will be, 's'.
The concentration of
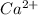 ion = s
ion = s
The concentration of
 ion = 2s
ion = 2s
The expression for solubility constant for this reaction will be,
![K_(sp)=[Ca^(2+)][OH^-]^2](https://img.qammunity.org/2020/formulas/chemistry/college/7nky2s7c80f0u39z9mkn8ij7t0zchfjo9y.png)
Let the solubility will be, 's'
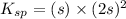
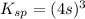
Now put the value of
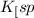 in this expression, we get the solubility.
in this expression, we get the solubility.
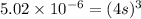
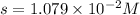
The concentration of
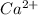 ion = s =
ion = s =
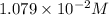
The concentration of
 ion = 2s =
ion = 2s =
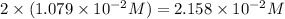
First we have to calculate the pOH.
![pOH=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/h1t4ubcsdqvqg0xpalkkvnwrun04y9pzd8.png)
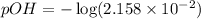
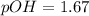
Now we have to calculate the pH.
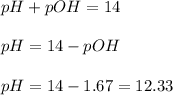
Therefore, the pH of a saturated solution is, 12.33