Answer:
180.04 nm
Step-by-step explanation:
λ₀ = maximum wavelength for photoelectric emission in tungsten = 230 x 10⁻⁹ m
E₀ = maximum energy of ejected electron = 1.5 eV = 1.5 x 1.6 x 10⁻¹⁹ J
λ = wavelength of light used = ?
Using conservation of energy
Energy of the light used = Maximum energy required for photoelectric emission + Energy of ejected electron
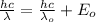
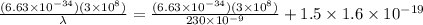
λ = 180.04 x 10⁻⁹ m
λ = 180.04 nm