Answer:
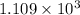 with four significant digits.
with four significant digits.
Step-by-step explanation:
Given,
 = 178.5 KJ/mole = 178500 J/mole (1kJ=1000J)
= 178.5 KJ/mole = 178500 J/mole (1kJ=1000J)
 = 161.0 J/mole.K
= 161.0 J/mole.K
According to Gibbs–Helmholtz equation:
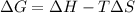
 = Gibbs free energy
= Gibbs free energy
 = enthalpy change
= enthalpy change
 = entropy change
= entropy change
T = temperature in Kelvin
 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 = -ve, reaction is spontaneous
= -ve, reaction is spontaneous
 = 0, reaction is in equilibrium
= 0, reaction is in equilibrium
As per question the reaction is spontaneous that means the value of
 is negative or we can say that the value is less than zero.
is negative or we can say that the value is less than zero.
Thus
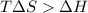
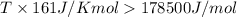
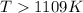
Significant figures are the figures in a number which express the value -the magnitude of a quantity to a specific degree of accuracy is known as significant digits.
Thus the temperature is
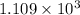 in kelvins above which this reaction is spontaneous.
in kelvins above which this reaction is spontaneous.