Answer:
Resonance structures for the nitrate ion exist because there are more than one Lewis Structures for this ion.
Step-by-step explanation:
How many bonds in each nitrate ion?
To answer this question, start by considering how many electrons each atom need for an octet.
- Each N atom needs three electrons to achieve an octet.
- Each O atom need two electrons to achieve an octet.
The three O atoms and one N atom in each nitrate ion will need
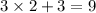 electrons in total to be stable. However, the ion carries a charge of
electrons in total to be stable. However, the ion carries a charge of
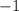 . In other words, atoms in the ion have already acquired one extra electron. Now they need only
. In other words, atoms in the ion have already acquired one extra electron. Now they need only
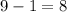 electrons.
electrons.
Atoms share electrons by forming bonds. In effect, each chemical bond (a pair of shared electrons) adds two electrons to the bonding atoms. Atoms in the nitrate ion will form
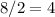 chemical bonds.
chemical bonds.
However, there are only three bonding position available. One of the three positions will see a double bond while each of the other two will see a single bond. However, the double bond can be at any of the three bonding position. There are thus three possible Lewis Structures. See the sketched. Note that the three structures are interconvertible by moving only the electrons but not any atoms. Hence the name resonance structures.
Keep in mind that in reality, the pi electrons from the double bond are delocalized across all three possible bonding positions. All three N-O bonds are of equal length.