Answer: The balanced chemical equation is written below.
Step-by-step explanation:
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side must be equal to the total number of individual atoms on the product side.
For the reaction of carbon dioxide with hydrogen gas, 9.85 kcal of energy is absorbed. So, this energy term will be written on the reactant side.
Thus, the balanced chemical equation for the reaction of carbon dioxide with hydrogen gas follows:
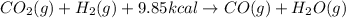
Hence, the balanced chemical equation is written above.