Answer : The equilibrium constant
 for the reaction is, 0.1133
for the reaction is, 0.1133
Explanation :
First we have to calculate the concentration of
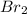 .
.
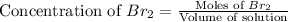
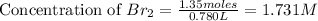
Now we have to calculate the dissociated concentration of
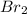 .
.
The balanced equilibrium reaction is,
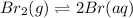
Initial conc. 1.731 M 0
At eqm. conc. (1.731-x) (2x) M
As we are given,
The percent of dissociation of
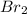 =
=
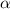 = 1.2 %
= 1.2 %
So, the dissociate concentration of
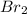 =
=
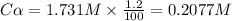
The value of x = 0.2077 M
Now we have to calculate the concentration of
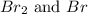 at equilibrium.
at equilibrium.
Concentration of
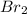 = 1.731 - x = 1.731 - 0.2077 = 1.5233 M
= 1.731 - x = 1.731 - 0.2077 = 1.5233 M
Concentration of
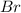 = 2x = 2 × 0.2077 = 0.4154 M
= 2x = 2 × 0.2077 = 0.4154 M
Now we have to calculate the equilibrium constant for the reaction.
The expression of equilibrium constant for the reaction will be :
![K_c=([Br]^2)/([Br_2])](https://img.qammunity.org/2020/formulas/chemistry/college/h0bn00cflpyehv4x63tgh7bv5vpzju2jc8.png)
Now put all the values in this expression, we get :
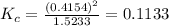
Therefore, the equilibrium constant
 for the reaction is, 0.1133
for the reaction is, 0.1133