Answer: oxidizing agent:

reducing agent:
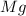
Step-by-step explanation:
Oxidation-reduction reaction or redox reaction is defined as the reaction in which oxidation and reduction reactions occur simultaneously.
Oxidation reaction is defined as the reaction in which a substance looses its electrons. The oxidation state of the substance increases.
Reduction reaction is defined as the reaction in which a substance gains electrons. The oxidation state of the substance gets reduced.
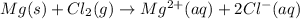
On reactant side:
Oxidation state of magnesium = 0
Oxidation state of chlorine = 0
On product side:
Oxidation state of magnesium = +2
Oxidation state of chlorine = -1
The oxidation state of chlorine reduces from 0 to -1, it is getting reduced. The substance which gets reduced, oxidize others and called as oxidizing agent.
The oxidation state of magnesium increases from 0 to +2. Thus, it is getting oxidized. The substance which gets oxidized, reduces others and called as reducing agent.