Answer:
The osmolarity of a solution is 0.003
Step-by-step explanation:
Osmolarity may be defined as the the number of solutes per volume of solution in litres.
The osmolarity of a solution can be calculated as follows:
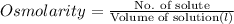
Here, No. of moles = 3 millimoles= 0.003 moles and volume = 1 liter.
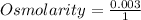
=0.003.
Hence, the osmolarity of a solution is 0.003.