Answer:
8.16%
Step-by-step explanation:
We are to calculate the mass percent of hydrogen in Methyl Acetate.
We know that the formula of Methyl Acetate is
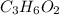 so firstly we will find its molecular mass.
so firstly we will find its molecular mass.
Molecular mass of (
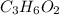 ) =
) =
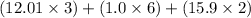 = 74.08 g
= 74.08 g
Mass of Hyrdogen in (
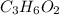 ) =
) =
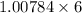 = 6.05 g
= 6.05 g
Mass percent of Hydrogen in Methyl Acetate =
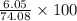 = 8.16%
= 8.16%