Answer: The excess reagent for a given reaction is nitrogen dioxide.
Step-by-step explanation:
Limiting reagent is defined as the reagent which is present in less amount and also it limits the formation of products.
Excess reagent is defined as the reagent which is present in large amount.
For the given chemical reaction:
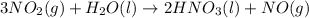
By stoichiometry of the reaction:
1 mole of water reacts with 3 moles of nitrogen dioxide gas
So, 0.5 moles of water will react with =
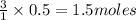 of nitrogen dioxide gas.
of nitrogen dioxide gas.
As, the given amount of nitrogen dioxide gas is more than the required amount. Thus, it is considered as an excess reagent.
Hence, nitrogen dioxide is an excess reagent.