Answer: The theoretical yield of iron (II) oxide is 5.53g and percent yield of the reaction is 93.49 %
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 ....(1)
....(1)
Given mass of iron = 4.31 g
Molar mass of iron = 53.85 g/mol
Putting values in above equation, we get:
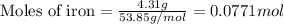
For the given chemical reaction:
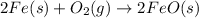
By Stoichiometry of the reaction:
2 moles of iron produces 2 moles of iron (ii) oxide.
So, 0.0771 moles of iron will produce =
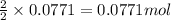 of iron (ii) oxide
of iron (ii) oxide
Now, calculating the theoretical yield of iron (ii) oxide using equation 1, we get:
Moles of of iron (II) oxide = 0.0771 moles
Molar mass of iron (II) oxide = 71.844 g/mol
Putting values in equation 1, we get:
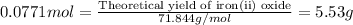
To calculate the percentage yield of iron (ii) oxide, we use the equation:
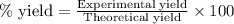
Experimental yield of iron (ii) oxide = 5.17 g
Theoretical yield of iron (ii) oxide = 5.53 g
Putting values in above equation, we get:
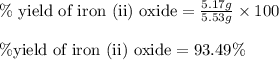
Hence, the theoretical yield of iron (II) oxide is 5.53g and percent yield of the reaction is 93.49 %