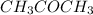Answer: A. -COO-
Step-by-step explanation:
Functional groups are specific group of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules.
A. Esters have functional group
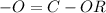 .
.
Example: methyl ethanoate with molecular formula
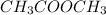
B. Aledydes have functional group
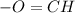 .
.
Example: Ethanal with molecular formula
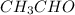
C. Acids have functional group
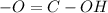 .
.
Example: Ethanoic acid with molecular formula
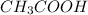
D. Ketones have functional group
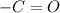 .
.
Example: Propanone with molecular formula