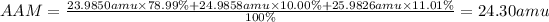Answer:
The average atomic mass is 24.30 amu.
Step-by-step explanation:
When there are at least 2 isotopes for an atom, each with its own atomic mass, we can calculate an average atomic mass (AAM), which considers the mass of each isotope and its relative abundance. The mathematical expression is:
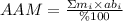
where,
AAM is the average atomic mass
mi is the mass of each isotope
abi is the relative abundance of each isotope
If we replace this expression with the data we have, then: