Step-by-step explanation:
As the given electronic configuration is
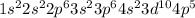 . This shows that there are in total 35 electrons and 7 valence electrons.
. This shows that there are in total 35 electrons and 7 valence electrons.
Also, we know that bromine is the element that has atomic number 35. And, since it belongs to group 17 so it has 7 valence electrons.
Thus, we can conclude that the given element is bromine.