Answer:
No
Step-by-step explanation:
Let's assume the gas pressure is constant. Then we can use Charle's law, which states that the volume of the gas is proportional to the absolute temperature (in Kelvin):
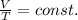
This can be rewritten as
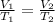
where we have
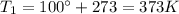 is the initial temperature of the gas
is the initial temperature of the gas
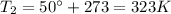 is the final temperature of the gas
is the final temperature of the gas
Re-arranging the previous equation, we find
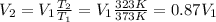
so, the volume of the gas decreases by a factor 0.87.