Answer:
Step-by-step explanation:
The given reaction equation is:
2A + 4B → C + 3D
We know the mass of compound A in the reaction above. We are to find the mass of compound D.
We simply work from the known mass to calculate the mass of the unkown compound D
Using the mole concept, we can find the unknown mass.
Procedures
- We first find the molar mass of the compound A from the atomic units of the constituent elements.
- We then use the molar mass of A to calculate its number of moles using the expression below:
Number of moles of A =
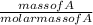
- Using the known number of moles of A, we can work out the number of moles of D.
From the balanced equation of the reaction, it is shown that:
2 moles of compound A was used up to produced 3 moles of D
Then
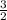 x number of moles of A would give the number of moles of D
x number of moles of A would give the number of moles of D
- Now that we know the number of moles of D, we can find its mass using the expression below:
Mass of D = number of moles of D x molar mass of D