Answer:
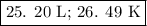
Step-by-step explanation:
25. Boyle's Law
The temperature and amount of gas are constant, so we can use Boyle’s Law.
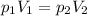
Data:
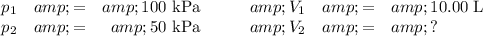
Calculations:
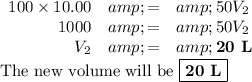
26. Ideal Gas Law
We have p, V and n, so we can use the Ideal Gas Law to calculate the volume.
pV = nRT
Data:
p = 101.3 kPa
V = 20 L
n = 5 mol
R = 8.314 kPa·L·K⁻¹mol⁻¹
Calculation:
101.3 × 20 = 5 × 8.314 × T
2026 = 41.57T
