Step-by-step explanation:
So,when balloon filled with helium gas was placed in lower temperature we will observe that the volume of the helium gas inside the helium will decrease due to which size of the balloon will also get decreased.
This is because volume is directly proportional to the temperature of the gas at constant pressure and number of moles. And this law is known as Charles law of
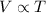 (At constant pressure and number of moles)
(At constant pressure and number of moles)