Answer:
4510 J
Step-by-step explanation:
To calculate the energy of the water, you need to use the following equation:
Q = mcΔT
In this equation,
-----> Q = energy/heat (J)
-----> m = mass (g)
-----> c = specific heat capacity (J/g°C)
-----> ΔT = change in temperature (°C)
First, you need to determine the mass of the water. To do this, you need to multiply the given volume by the density of water (1.00 g/mL).
100.0 mL H₂O 1.00 g
------------------------ x ------------ = 100.0 g H₂O
1 mL
Now, you can plug the given values into the equation and solve for "Q" (Q =
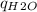 ). The final answer should have 3 significant figures to match the given values with the lowest number of sig figs.
). The final answer should have 3 significant figures to match the given values with the lowest number of sig figs.
Q = ? J c = 4.18 J/g°C
m = 100.0 g ΔT = 32.0 °C - 21.2 °C = 10.8 °C
Q = mcΔT
Q = (100.0 g)(4.18 J/g°C)(10.8 °C)
Q = 4510 J