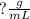Answer:
Step-by-step explanation:
You can determine the units in the final answer by identifying which of the units cancel out. Units are eliminated (cancelled out) when they are located both in the numerator and denominator of proportions being multiplied.
In this case, these units are cancelled out.....
-----> milligrams (mg) = (1st and 2nd proportions)
-----> decaliters (dL) = (1st and 3rd proportions)
-----> liters (L) = (3rd and 4th proportions)
As these units are not cancelled out, you are left with grams (g) in the numerator and milliliters (mL) in the denominator.
The final units should be represented by: