Hello!
The answer is:
The density is equal to:
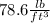
Why?
This a unit conversion problem, so, converting the density of glycerin from
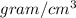 to
to
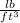 using the given conversion rates, we have:
using the given conversion rates, we have:
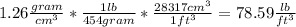
Rounding to three significant figures, we have that the density is equal to:
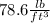
Have a nice day!