Hello!
The answer is:
The new volume is equal to 206.5 L.
Why?
To solve this problem, we need to assume that the pressure is constant, and use the Charle's Law equation, so, solving we have:
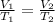
We are given:
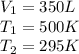
Then, using the Charle's Law equation, we have:
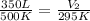
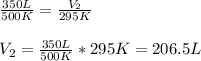
Hence, we have that the new volume is equal to 206.5 L.
Have a nice day!