Answer:
29 mL
Step-by-step explanation:
Equation
The question needs us to find the volume of the liquid. The equation for volume using density and mass is:
Volume = Mass / Density
Solve
We can substitute the given values for density and mass into the equation:
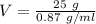
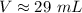
Additional Comments
The answer we obtained (29 mL) is rounded to two significant figures. When multiplying or dividing, the amount of significant figures in the final answer is always the least amount of significant figures in one of the values.
Below are the significant figure rules:
Nonzero digits will always be significant (eg. 54 --> 2 significant figures)
Zeroes at the beginning of a number will never be significant (eg. 0.1 --> 1 significant figure)
Zeroes between two nonzero digits will always be significant (eg. 504 --> 3 significant figures)
Zeroes following a number will always be significant if the number contains a decimal point (eg. 40.0 --> 3 significant figures)