Answer:
2.60 L
Step-by-step explanation:
To find the new volume, you need to use the Combined Gas Law:
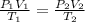
In this equation, "P₁", "V₁", and "T₁" represent the initial pressure, volume, and temperature. "P₂", "V₂", and "T₂" represent the final pressure. volume, and temperature.
At STP, the pressure is 1.0 atm and the temperature is 273 K. Before you can plug the values into the equation, you need to convert pressure from torr to atm and the temperature from Celsius to Kelvin.
P₁ = 720 torr / 760 = 0.947 atm P₂ = 1.0 atm
V₁ = 3.0 L V₂ = ? L
T₁ = 25 °C + 273 = 298 K T₂ = 273 K
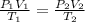 <----- Combined Gas Law
<----- Combined Gas Law
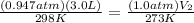 <----- Insert values
<----- Insert values
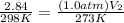 <----- Multiply 0.947 and 3.0
<----- Multiply 0.947 and 3.0
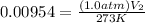 <----- Simplify left side
<----- Simplify left side
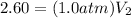 <----- Multiply both sides by 273
<----- Multiply both sides by 273
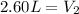 <----- Divide both sides by 1.0
<----- Divide both sides by 1.0