Answer:
Because the object should shrink its volume to zero, which is impossible
Step-by-step explanation:
Let's talk about gases for simplicity. Ideal gases are governed by the ideal gas equation:
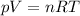
where
p is the gas pressure
V is the volume of the gas
n is the number of moles
R is the gas constant
T is the absolute temperature
From the formula, we see that T and V are directly proportional: therefore, in order for a gas to have an absolute temperature of zero, it must also have a volume of zero, which is impossible.