Answer : The time taken for the reaction is 36363.64 minutes.
Explanation :
First we have to convert the mass of iron oxide from milligram to pounds (lbs).
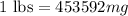
or,
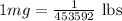
As,
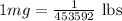
So,
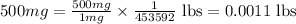
Now we have to calculate the time taken for the reaction.
As,
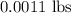 of iron oxide take time = 20 minutes
of iron oxide take time = 20 minutes
As,
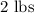 of iron oxide take time =
of iron oxide take time =
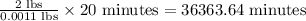
Therefore, the time taken for the reaction is 36363.64 minutes.