Answer:
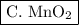
Step-by-step explanation:
2H₂O₂ ⟶ 2H₂O + O₂
A catalyst is a substance that speeds up a reaction but can be recovered unchanged at the end.
Thus, at the end of the reaction I would expect to find H₂O and MnO₂ in the container.
Water is not listed in any of the options, so I will have to choose
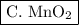 .
.
A, B, and D are wrong. They are not reactants, products, or catalysts.