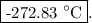Answer:
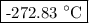
Step-by-step explanation:
We can use the Ideal Gas Law to calculate the temperature.
pV = nRT
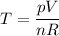
Data:
p = 0.998 atm
V = 0.125 L
n = 4.75 mol
R = 0.082 06 L·atm·K⁻¹mol⁻¹
Calculation:
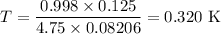
T = (0.320 – 273.15) °C = -272.83 °C
Note: This is an impossible situation. CO₂ solidifies at -78.5 °C.
If CO₂ were an ideal gas, the calculated temperature would be