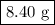Answer:
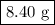
Step-by-step explanation:
The half-life of K-40 (1.3 billion years) is the time it takes for half of it to decay.
After one half-life, half (50 %) of the original amount will remain.
After a second half-life, half of that amount (25 %) will remain, and so on.
We can construct a table as follows:
No. of Fraction
half-lives t/yr Remaining
0 0 1
1 1.3 billion ½
2 2.6 ¼
3 3.9 ⅛
We see that after 2 half-lives, ¼ of the original mass remains.
Conversely, if two half-lives have passed, the original mass must have been four times the mass we have now.
Original mass = 4 × 2.10 g =