Answer:
0.0757 M
Step-by-step explanation:
The question asks for the new concentration of a solution after being diluted. For this we can use the dilution formula:
M₁ V₁ = M₂ V₂
Where M stands for molarity and V for volume, so M₁ and V₁ are the initial values for molarity and volume, and M₂ and V₂ are the final values of molarity and concentration.
So
M₁ = 0.455 M
V₁ = 375 mL = 0.375 L (because 1 L = 1000 mL)
The question says the solution is diluted with 1.88 L of water, so to the initial 375 mL of solution, 1.88L of water are added, hence the final volume is:
V₂ = 0.375 L + 1.88L = 2.255 L
M₂ is the final concentration which we want to calculate, so from the formula we get:
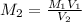
M₂ = (0.455 M × 0.375 L) / 2.255 L = 0.0757 M
The new concentration after the dilution is 0.0757 M