Answer:
Choice A: carbon-12,
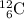 .
.
Step-by-step explanation:
- The number on the top-left corner of each particle is its mass number.
- The number on the lower-left corner of each particle is its atomic number.
Let
- the mass number of X be A, and
- the atomic number (a.k.a. proton number) of X be Z.
Symbol of X:
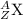 .
.
Consider the two conservation laws:
- Sum of mass numbers on the left-hand side = Sum of mass numbers on the right-hand side.
- Sum of atomic numbers on the right-hand side = Sum of atomic numbers on the right-hand side.
Sum of mass numbers:
- Left-hand side: 9 + 4;
- Right-hand side: A + 1.
Equate the two sides:

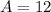 .
.
Thus the mass number of X is 12.
Try the steps above to find the atomic number of X. The number
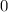 on the lower-left corner of
on the lower-left corner of
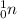 indicates that the atomic number of this neutron is zero.
indicates that the atomic number of this neutron is zero.
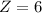 .
.
Thus the atomic number of X is 6.
What element is X? Refer to a modern periodic table. The element with atomic number 6 is carbon. The symbol for carbon is
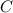 . The mass number of this isotope is 12. The answer will be
. The mass number of this isotope is 12. The answer will be
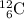 .
.