Answer:
The atomic number increases by 1.
Step-by-step explanation:
The beta minus decay is a process in which a neutron decays into a proton, emitting an electron and an anti-neutrino:
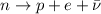
If this process occurs inside an unstable nucleus, we notice that:
- a neutron is converted into a proton, therefore
- the number of neutrons decreases by 1 and the number of protons increases by 1
Keep in mind that the atomic number of a nucleus corresponds to the number of protons it contains: therefore, since this number increases by 1, then the atomic number increases by 1.