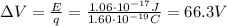Answer:
66.3 V
Step-by-step explanation:
The wavelength of the electron must be equal to that of the x-ray photon:
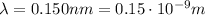
the De Broglie wavelength of the electron is related to its momentum, p, by the formula
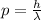
where h is the Planck constant. Solving the formula, we find
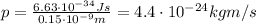
Now we can find the electron's energy using the formula
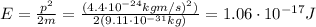
Then, we know that the energy of an electron accelerated through a potential difference of
 is
is
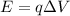
where
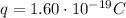 is the electron charge
is the electron charge
Solving the equation for the potential difference, we find