Answer:
247 molecules
Step-by-step explanation:
This problem can be solved by using the ideal gas equation:
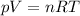
where in this case we have
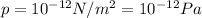 is the lowest pressure attainable
is the lowest pressure attainable
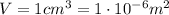 is the volume we are considering
is the volume we are considering
n is the number of moles
R is the gas constant
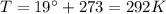 is the absolute temperature
is the absolute temperature
Solving the equation for n, we find
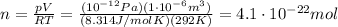
And since the number of molecules in 1 mole of gas is
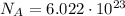 (avogadro number)
(avogadro number)
The number of molecules present here is
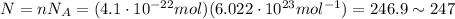
so, there are approximately 247 molecules.