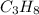Answer:
The correct answer option is 0.883 moles
Step-by-step explanation:
We are to find the number of moles that are consumed when a propane heater burns 38.95 g
 .
.
For this, we will divide the mass (38.95 g) of propane (
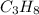 ) by the molar mass which is:
) by the molar mass which is:
C = 12.01
H = 1.01
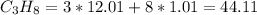
Dividing the mass by 44.11 to get the number of moles consumed:
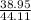 = 0.883 moles
= 0.883 moles