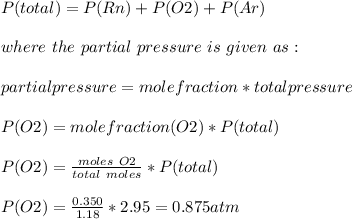
Answer:
Partial pressure of O2 = 0.875 atm
Step-by-step explanation:
Given:
Moles of Radon gas (Rn) =0.220
Moles of O2 gas = 0.350
Moles of argon gas (Ar) = 0.640
Total pressure = 2.95 atm
To determine:
The partial pressure of O2
Step-by-step explanation:
As per Dalton's Law, the total pressure exerted by a mixture of gases is equal to the sum of their partial pressures.
Here: