Hello!
The answer is: The new pressure is 1.67 atm.
Why?
From the statement, we know that the temperature remains constant and the gas volume is changing, meaning that the new pressure will be different than the first pressure.
Since the temperature remains constant, we can calculate the new pressure using the Boyle's Law.
The Boyle's Law states that:
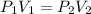
Where,
P is the pressure of the gas.
V is the volume of the gas.
Then, the given information is:
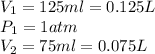
Remember, 1 L is equal to 1000 mL.
So,
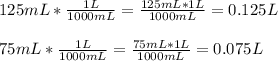
So, calculating the new volume, we have:
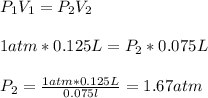
Hence, the new pressure is 1.67 atm.
Have a nice day!