Answer:
The temperature of the substance with the lower specific heat will increase more than the one with a higher specific heat
Step-by-step explanation:
The increase of temperature of a substance is given by:
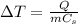
where
Q is the amount of heat absorbed by the substance
m is the mass of the substance
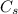 is the specific heat capacity of the substance
is the specific heat capacity of the substance
In this problem, we have two substances with same mass m, that absorb the same amount of heat Q. We see from the formula that the increase in temperature is inversely proportional to the specific heat: therefore, the substance with lower specific heat will have a greater increase in temperature than the substance with higher specific heat.