Answer:
Step-by-step explanation:
The half-life time of a radiactive isotope (radioisotope) is a constant value, meaning that the amount of the radioisotope that decays will be (1/2) raised to the number of half-lives passed.
Naming A₀ the initial amount to the radioisotope, you can build this table to find the amount left.
Number of half-lives amount of radiosotope left
0 A₀
1 (1/2) × A₀
2 (1/2)×(1/2)×A₀ = (1/2)² × A₀
3 (1/2)³ ×A ₀
4 (1/2)⁴ × A₀
n (1/2)ⁿ × A₀
Now calculate the number of half-lives the strontium-90 sample has passed after 100 years:
- n = 100 years / 28.1 years ≈ 3.5587
Hence, the amount of strontium-90 is:
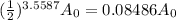
In percent, that is:
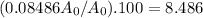
Rounding to two significant figures, that is 8.5%.
Conclusion: The percent of strontium-90 left after 100 yeaers is 8.5% (choice number 4).