Answer:
Choice A.
Step-by-step explanation:
The motion of molecules in a system depends on the amount of kinetic energy in the system. However, the kinetic energy of a system is part of its internal energy. Assume that all other components of the internal energy stay the same. The increase in the motion of molecules will be the greatest in the system with the greatest increase in internal energy.
Which choice will increase the internal energy of the system the most?
The change in internal energy of a system is the sum of the heat added to the system and the work done on the system. In other words,
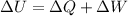 ,
,
where
 is the gain in the internal energy of the system.
is the gain in the internal energy of the system.
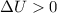 means that there's an increase in the internal energy of the system. That might be a sign that the molecular motion in the system increases. On the other hand,
means that there's an increase in the internal energy of the system. That might be a sign that the molecular motion in the system increases. On the other hand,
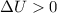 shows a decrease in the internal energy of the system. The molecular motion in the system might decrease.
shows a decrease in the internal energy of the system. The molecular motion in the system might decrease.
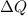 is the heat added to the system.
is the heat added to the system.
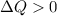 if overall the system absorbs heat.
if overall the system absorbs heat.
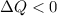 if overall the system releases heat.
if overall the system releases heat.
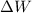 is the work done on the system.
is the work done on the system.
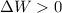 if overall work is done on the system.
if overall work is done on the system.
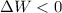 if overall the system does work on its surroundings.
if overall the system does work on its surroundings.
Consider each choice:
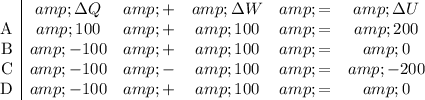 .
.
Choice A gives the greatest increase in the internal energy of the system. Note that the kinetic energy of the molecules is only one of the many components of internal energy. For example, there might be a change in state (e.g., melting) that changes the internal energy without changing the kinetic energy. Assume that all other components stay the same in all four choices. The average molecular motion will increase the most in option A.