Expected:
sp³ in all three molecules.
Step-by-step explanation
The hybridization of the central atom is related to the number of electron domains around that atom.
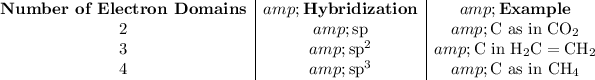 .
.
What is an electron domain?
- An atom bonded to the central atom counts as one electron domain. That atom counts as one electron domain regardless of the bond order. One single bond counts as one electron domain. One double bond counts as one electron domain. One triple bond counts as one electron domain.
- A lone pair of electrons count as one electron domain.
How many electron domains in BH₄⁻, CH₄, and NH₄⁺?
- BH₄⁻: Four H atoms are bonded to the central B atom. That ensures an octet for the central B atom. No lone pairs are needed. Four electron domains from the four bonded atoms. sp³ hybridization.
- CH₄: Four electrons domains with four H atoms and no lone pair. sp³ hybridization.
- NH₄⁺: Four electrons with four H atoms and no lone pair. sp³ hybridization.